Draw the most stable lewis structure of acrolein – Embark on a captivating journey to uncover the secrets of acrolein’s molecular structure. Delving into the realm of Lewis structures, we embark on a quest to identify the most stable configuration, unraveling the intricate interplay of resonance, electronegativity, and hybridization.
Prepare to witness the dance of electrons, as we decipher the molecular formula, unravel the intricacies of structural formulas, and delve into the profound significance of resonance structures. The quest for stability beckons, guiding us towards the most enduring arrangement of atoms and bonds.
Molecular Structure of Acrolein
Acrolein, also known as propenal, is an organic compound with the molecular formula C 3H 4O. It is a colorless liquid with a pungent odor. The structural formula of acrolein can be represented as CH 2=CH-CHO, while its skeletal formula is C=C-C=O.
Resonance Structures of Acrolein
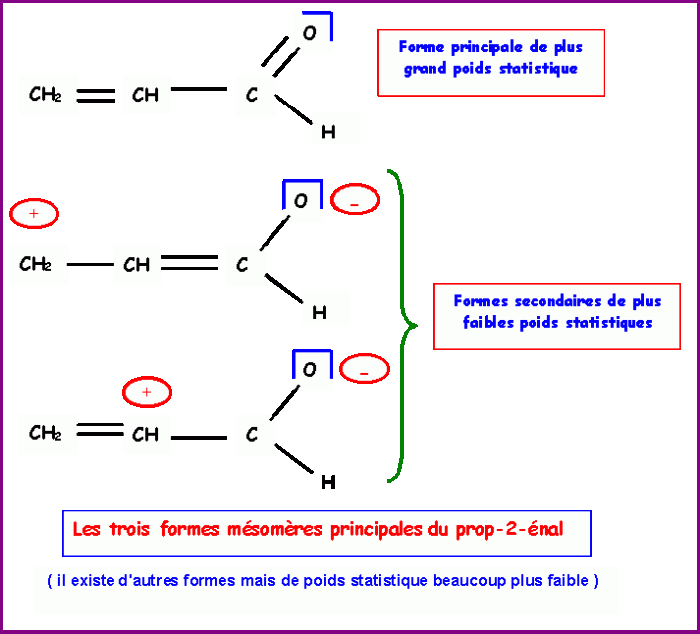
Resonance is a concept in chemistry that describes the delocalization of electrons within a molecule. In the case of acrolein, there are two possible resonance structures:
- CH 2=CH-CHO
- CH 2-CH=O
The first resonance structure is more stable than the second because the negative charge is located on the more electronegative oxygen atom.
Formal Charges and Electronegativity
Formal charges are a way of assigning charges to atoms in a molecule. The formal charge on an atom is calculated by subtracting the number of electrons assigned to the atom in the Lewis structure from the number of valence electrons the atom has in its elemental state.
The electronegativity of an atom is a measure of its ability to attract electrons. The more electronegative an atom, the more likely it is to have a negative formal charge.
In the case of acrolein, the formal charges on the atoms are as follows:
- Carbon 1: 0
- Carbon 2: 0
- Oxygen: -1
The most stable resonance structure is the one with the lowest formal charges. In this case, the most stable resonance structure is CH 2=CH-CHO.
Hybridization and Bonding: Draw The Most Stable Lewis Structure Of Acrolein
The carbon atoms in acrolein are sp 2hybridized. This means that they have three hybrid orbitals that are used to form sigma bonds with the other atoms in the molecule. The fourth valence electron on each carbon atom is used to form a pi bond with the other carbon atom.
The sigma bonds in acrolein are formed by the overlap of sp 2hybrid orbitals. The pi bond is formed by the overlap of two p orbitals.
The geometry of acrolein is trigonal planar. This is because the three sp 2hybrid orbitals on each carbon atom are arranged in a trigonal plane.
Molecular Orbitals and Delocalization
The molecular orbitals of acrolein can be described using molecular orbital theory. Molecular orbital theory is a method for calculating the electronic structure of molecules. It is based on the idea that the electrons in a molecule are delocalized over the entire molecule.
The pi-bond system in acrolein is delocalized. This means that the electrons in the pi-bond system are not localized to a single bond. Instead, they are spread out over the entire pi-bond system.
The delocalization of the pi-bond system in acrolein makes the molecule more stable. This is because the delocalization of the electrons lowers the energy of the molecule.
Query Resolution
What is the molecular formula of acrolein?
C 3H 4O
How many resonance structures does acrolein have?
Two
Which resonance structure of acrolein is more stable?
The one with the negative charge on the oxygen atom