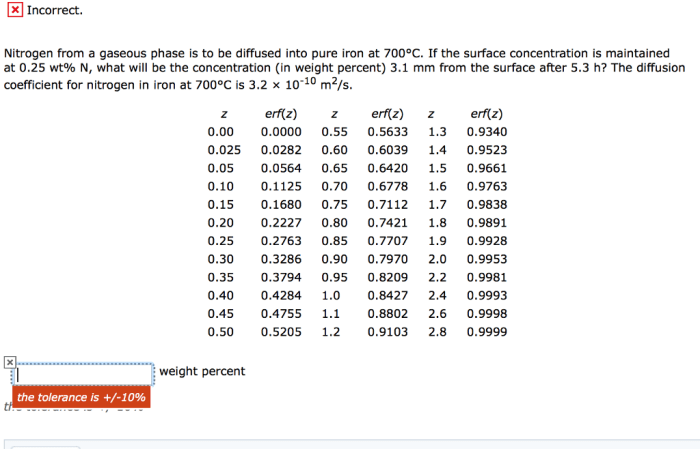
Nitrogen from a gaseous phase is to be diffused – Nitrogen diffusion from a gaseous phase plays a critical role in numerous industrial and scientific applications. Understanding the mechanisms, measurement techniques, and modeling approaches associated with nitrogen diffusion is essential for optimizing these applications and unlocking new possibilities.
This comprehensive overview explores the molecular processes involved in nitrogen diffusion, discusses experimental methods for measuring diffusion coefficients, and examines mathematical models used to simulate nitrogen diffusion behavior. Furthermore, it highlights industrial applications where nitrogen diffusion is crucial and discusses its role in scientific research.
Nitrogen Diffusion Mechanisms
Nitrogen diffusion involves the molecular movement of nitrogen gas from an area of high concentration to an area of low concentration. This process occurs through several mechanisms, including molecular diffusion, Knudsen diffusion, and surface diffusion.
Molecular diffusion is the primary mechanism responsible for nitrogen diffusion in the gaseous phase. It involves the random motion of nitrogen molecules, which collide with each other and travel in various directions. The net movement of these molecules is from an area of high concentration to an area of low concentration.
Knudsen diffusion is a type of molecular diffusion that occurs when the mean free path of nitrogen molecules is comparable to or greater than the pore size of the material through which they are diffusing. In this case, the molecules move in a more directed manner, colliding with the pore walls rather than with each other.
Surface diffusion occurs when nitrogen molecules adsorb onto the surface of a material and move along the surface. This mechanism is typically less significant than molecular diffusion and Knudsen diffusion but can be important in certain applications.
Factors Influencing Nitrogen Diffusion Rates
- Temperature: Higher temperatures increase the kinetic energy of nitrogen molecules, leading to faster diffusion rates.
- Pressure: Increased pressure increases the number of nitrogen molecules per unit volume, leading to more frequent collisions and faster diffusion rates.
- Concentration gradient: A steeper concentration gradient between the two areas creates a greater driving force for diffusion, resulting in faster diffusion rates.
- Pore size: For Knudsen diffusion, smaller pore sizes restrict the movement of nitrogen molecules, leading to slower diffusion rates.
- Adsorption: Strong adsorption of nitrogen molecules onto the surface of a material can reduce the rate of surface diffusion.
Experimental Techniques for Measuring Nitrogen Diffusion

Various experimental techniques are used to measure nitrogen diffusion coefficients. These techniques can be classified into three main categories:
Steady-State Permeation
Steady-state permeation involves measuring the rate of nitrogen gas flow through a thin membrane that separates two chambers with different nitrogen concentrations. The diffusion coefficient is calculated from the flow rate and the membrane thickness.
Transient Diffusion
Transient diffusion involves measuring the change in nitrogen concentration over time in a closed chamber. The diffusion coefficient is calculated from the rate of concentration change and the geometry of the chamber.
Electrochemical Methods
Electrochemical methods involve measuring the current generated by the diffusion of nitrogen gas across an electrochemical cell. The diffusion coefficient is calculated from the current and the cell parameters.
The choice of experimental technique depends on factors such as the desired accuracy, the range of diffusion coefficients to be measured, and the availability of specialized equipment.
Modeling and Simulation of Nitrogen Diffusion: Nitrogen From A Gaseous Phase Is To Be Diffused
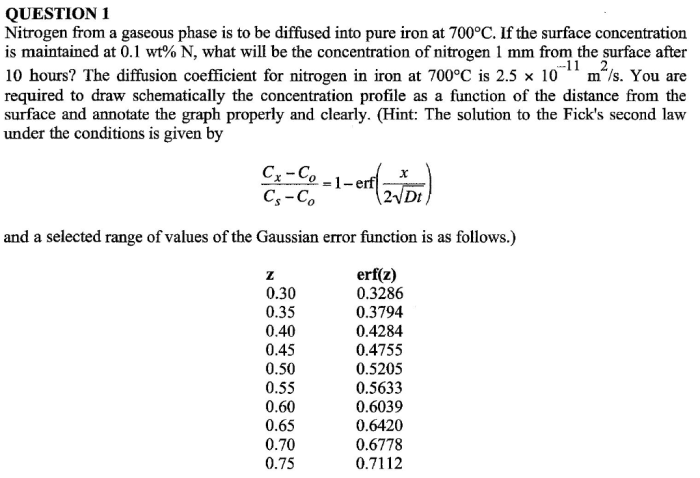
Mathematical models are used to simulate nitrogen diffusion in various systems. These models can be based on the Fick’s laws of diffusion or more complex computational fluid dynamics (CFD) simulations.
Fick’s laws of diffusion describe the diffusive flux of nitrogen gas as proportional to the concentration gradient. These laws can be used to develop analytical solutions for simple diffusion problems.
CFD simulations solve the governing equations of fluid flow and mass transfer numerically. These simulations can be used to model complex diffusion problems involving irregular geometries, multiple species, and non-isothermal conditions.
Assumptions and Limitations, Nitrogen from a gaseous phase is to be diffused
- Fick’s laws assume constant diffusion coefficients and isotropic diffusion.
- CFD simulations require accurate boundary conditions and mesh generation.
- Both approaches may not accurately capture the effects of surface diffusion or Knudsen diffusion in certain applications.
Applications of Nitrogen Diffusion in Industry and Research
Nitrogen diffusion plays a crucial role in various industrial and research applications:
Industrial Applications
- Chemical processing: Nitrogen is used as an inert gas in chemical reactions to prevent oxidation and explosion hazards.
- Gas separation: Nitrogen diffusion is used to separate nitrogen from other gases in air separation units and natural gas processing plants.
- Heat treatment: Nitrogen is used as a protective atmosphere in heat treatment processes to prevent oxidation and carburization of metals.
Scientific Research
- Gas transport in porous materials: Nitrogen diffusion is studied to understand the transport of gases in porous materials such as zeolites and activated carbon.
- Biological systems: Nitrogen diffusion is essential for understanding the transport of gases in biological systems, such as the exchange of oxygen and carbon dioxide in the lungs.
- Emerging fields: Nitrogen diffusion is being explored for applications in nanotechnology, microfluidics, and energy storage systems.
Questions Often Asked
What are the key factors that influence nitrogen diffusion rates?
Temperature, pressure, and concentration gradients are the primary factors that affect the rate of nitrogen diffusion.
How can nitrogen diffusion be measured experimentally?
Various experimental methods are available, including steady-state permeation, transient diffusion, and electrochemical methods.
What are the applications of nitrogen diffusion in industry?
Nitrogen diffusion plays a vital role in chemical processing, gas separation, and heat treatment.